Project 1. Aggregation of Amyloidogenic Proteins
Aging-related dementia, Alzheimer’s disease (AD) and Parkinson’s disease (PD), are among the leading causes of death in the developed countries. They not only threaten people’s life, more seriously, greatly lower the life quality of the patients. Many research findings have strongly suggested that deposition of alpha-synuclein in Lewy bodies in the form of alpha-synuclein fibrils contributes to the progression of PD. Similarly, senile plaques and neurofibrillary tangles, the hallmark of AD, are found to be composed of mainly aggregates/fibrils of variants of the beta-amyloid.
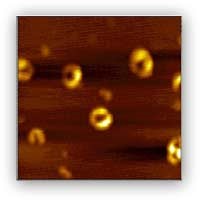
Given the similarity of AD and PD in that misfolding, aggregation, and deposition of amyloidogenic proteins or peptides, studying factors that may affect the aggregation process of alpha-synuclein and beta-amyloid is of general interest. In addition, it has been hypothesized that interaction of redox active metals with amyloid protein produces reactive oxygen species through a Fenton-like chemistry, and the attack of the reactive oxygen species on neurons and the subsequent deposition of amyloid protein lead to the damage of the neurons. Although several studies have been conducted that support this oxidative theory, the mechanism is still unclear. As such, our group is conducting studies on two fronts. One focuses on the kinetics of aggregation process of the study of beta-amyloid and alpha-synuclein and identification of intermediates (oligmers and protofibrils) at different aggregation stages under various conditions. Atomic force microscopy (Representative images shown in Figure 1) is one of the several major techniques used to study the aggregation process. On another front, we focus on how the interaction of metals affects the aggregation of beta-amyloid and alpha-synuclein in terms of both aggregation kinetics and aggregates structures, and to study the mechanism of production of reactive oxygen species through the interactions.
Figure 1. AFM image of annular aggregates/intermediates
Project 2. Electron and Metal Transfer Involving Metallothioneins
Metallothionein (MT), a protein involved in metabolism of essential metal ions and detoxification of environmentally accumulated metal ions, has 60 amino acids and binds seven metal ions in two domains formed independently at either end of the protein chain. The incorporation of metal ions such as Zn or Cu confers steric possibilities, catalytic activity, or redox properties to the acceptor metalloproteins to control important biological processes as varied as gene expression, respiration, and acid/base balance. It has been hypothesized that the thiolate clusters that coordinate the metal ions in MTs may render the molecule redox characteristics and can modulate the metal transfer between MT and a biological substrate. We have collaborated with researchers at CSU-Long Beach to quantify the amount of metal release using several hybrid instruments, such as electrochemistry combined on-line with inductively coupled plasma-mass spectrometry (EC/ICP-MS) and HPLC/ICP-MS. In addition, we have also elucidated the mechanism through which MT undergoes electron transfer at mercury film electrodes. Using nanoparticle-amplified voltammetric detections, we have also quantified the thiol groups present in MTs that are deficient in metals. We are currently investigating the metal transfer processes between MT and other biomolecules.
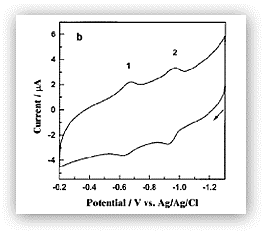
Figure 2. Cyclic voltammogram of rabbit liver MTs adsorbed onto a thin mercury film electrode. The two reversible redox waves correspond to the cysteine-mercury thiolate bond reactions and the formation of disulfide bonds within the surface-bound MT molecules.
Project 3. Biological Imaging at the Solution/Solid Interface
Developments of DNA and protein microarray (also referred to as biochip) technology are accelerating rapidly and the knowledge accumulated from years of fundamental research and practical applications related to heterogeneous DNA and protein biosensors is utterly essential. Thus, studies of the effect of immobilization methods and characterization of surface-confined biomolecules are critical to the development of effective heterogeneous biosensors and microarrays. This has been one of our focal points in the past few years. On another front, we are exploring various imaging methods for sensitive and high-throughput analysis of microarray surfaces. Although fluorescent labels yield extremely high sensitivity and are well-suited for multiplexing assays, fluorescence imaging has several inherent drawbacks (e.g., photobleaching and sample-labeling that may introduce ambiguities). We are exploring alternative approaches that are complementary to fluorescence imaging. These techniques include scanning electrochemical microscopy, imaging ellipsometry, and surface plasmon resonance (SPR) microscopy. We have recently acquired a microarray scanner and will conduct parallel measurements to compare the performances of these alternative imaging techniques.
Figure 3. An image of a DNA microarray spotted in house.
Project 4. Coupled Analytical Techniques
Developing coupled analytical techniques that are capable of performing sensitive, selective, in-situ, and real-time measurements has been a long-standing effort of our group. Below are three representative and recent examples in this research area.
a) Flow-Injection High-Resolution Surface Plasmon Resonance (SPR). A high-resolution surface plasmon resonance (SPR) has been successfully combined with a microbore flow-injection system (FI-SPR) and utilized for studies of biological interactions (e.g., DNA hybridization). The instrument is capable of measuring SPR dip shifts at 10-4degrees or less (Figure 4). We have also extended its applications to measurements of heavy metals, such as Cd(II) and Hg(II) at chemically and biologically modified SPR sensor surfaces, and enzyme- and nanoparticle-amplified gene and protein assays.
Figure 4. A setup of the flow-injection high-resolution surface plasmon resonance instrument. The instrument can also be readily coupled to electrochemistry.
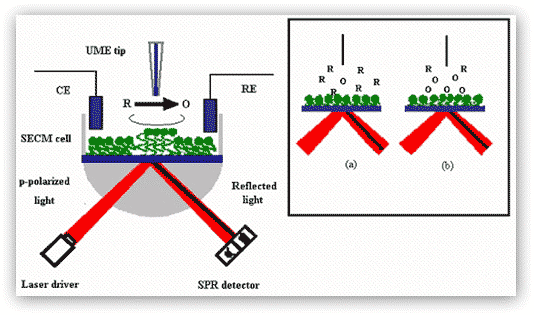
b) Scanning electrochemical microscopy-surface plasmon resonance (SECM-SPR):We have combined our high-resolution SPR with a scanning electrochemical microscope. Figure 5 illustrates the principle behind the SECM-SPR combination for sensitive measurements of film thickness variation and orientation changes of surface-confined molecules in a highly localized area. The high sensitivity of the SPR facilitates the measurement of infinitesimal film thickness changes accompanying redox reactions, while the SECM provides a means to obviate the necessity of applying a potential to the SPR substrate, which tends to cause unwanted interferences and complications. The approach also affords an avenue for determining film thickness variations that are not subject to certain effects, such as the surface charge, the heterogeneity of the substrate, and the distance between the redox center of the immobilized molecule and the underlying substrate electrode.
Figure 5. SECM-SPR combination for studies of redox-induced, localized film thickness variation and molecular orientation changes. Oxidation of redox moieties at the film by a tip-generated oxidant (O) is used as an example. The SECM cell containing the counter electrode (CE), the reference electrode (RE), and the UME tip is mounted directly onto of the SPR prism and instrument. Inset: schematic representations of the diffusion zones at the moment when O is generated at the tip (a) and at the time when O is produced at the diffusion-controlled rate (b). Notice that the position of the SPR dip (dark line) has changed from (a) to (b) upon the molecular orientation change.
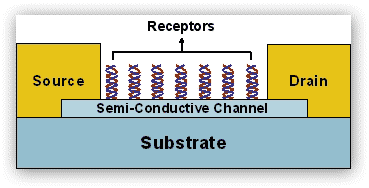
Project 5. Nanomaterials and Nanodevices for Enhanced Biosensing
Chemically and biologically sensitive resistors (chemiresistors) are currently leading candidates for vapor detection of environmentally and biologically important molecules. Since these sensors are simple to prepare and inherently compatible with conventional silicon electronics processing and readout circuitry, they can be miniaturized and tailored for different applications. The focus of our group has been on chemically sensitive resistors that utilize semi-conductive 1D material, such as individual or networks of single wall carbon nanotubes (SWCNTs) or semiconductor nanoparticle arrays or networks for detection of a wide variety of biological and chemical targets in both liquid and gas phases.
Figure 6. Idealized scheme depicting SWCNT-based chemiresistors with receptors, where binding of biomolecule with net electrical charge change the conductance in the channel. The channel can be either individual or network of SWCNTS.
In such structures, the 1D-structure provides the electric conductivity and the biomolecules provide sites for the selective sorption of analyte molecules. This approach has three main attributes: (1) the presumed ability to synthesize, if not at will, then with much control, nearly any type of hybrid structures; (2) enhanced sensitivity resulted from the network assembly of various structures; and (3) the versatility in modifying the binding through various processes (e.g., non-covalent, hydrogen bonding, coordination, etc.) and linker molecules and nanomaterials. By judicious choice of the building blocks, we can combine, in the same chemiresistor framework, two or more properties that are difficult to achieve in classical devices.