Welcome to the Gomez Group Homepage
Dr. Frank A. Gomez
Executive Director, STEM-NET, Department of Research
CSU Office of the Chancellor
401 Golden Shore, 6th Floor, Long Beach, CA 90802-4210
Tel. (562) 951-4774 / E-mail: [email protected]
Professor of Chemistry
The Gomez research group is engaged in developing fundamental and applied research in the area of microfluidics. Specifically, we are focused on developing new microfluidic platforms for point-of-care (POC) diagnostic devices, microfluidic batteries (MBs) and fuel cells (MFCs), and chemical and biochemical separations. Current work involves the development of paper microfluidics and enzyme-linked immunosorbent assays (ELISAs) on microfluidic platforms, electrochemical-based assays, enzyme microreactors, novel materials for microfluidics, and chromatography on chips. We also employ response surface methodology (RSM) and artificial neural networks (ANN) to experimentally optimize conditions in microfluidics. The members of the Gomez group include undergraduate and graduate students, high school students, postdoctoral fellows, and visiting scientists, from the fields of chemistry, biochemistry, mechanical and electrical engineering, physics, biology, and mathematics.
Frank A. Gomez
- Postdoc : Harvard, MA, 1991-1994 (with Prof. George. M. Whitesides)
- Ph.D. : UCLA, CA, 1991 (with Prof. M. Frederick Hawthorne)
- B.S. : Cal State, LA, CA, 1986 (with Prof. Thomas P. Onak)
Frank A. Gomez is the inaugural Executive Director of STEM-NET, the newest Multi-Campus Collaboration, also known as Affinity Group, at the CSU Office of the Chancellor. It potentially constitutes the most extensive affinity group since all 23 campuses will be members. The vision of STEM-NET is to make the CSU a worldwide leader in increasing the pipeline, preparation, graduation and employment of outstanding, diverse STEM students. The mission of STEM-NET is to enable CSU stem leaders to share expertise and leverage system-wide opportunities to foster the implementation of global best practices for our students and faculty in pedagogy, learning and research related to STEM fields within the CSU system
Gomez was Professor of Chemistry at Cal State LA since 1994 and was the university Faculty Research Liaison since 2014. He received his B.S. (1986) and Ph.D. (1991) in Chemistry from Cal State LA and UCLA, respectively. From 1991-1994 he was a Damon Runyon-Walter Winchell Cancer Research Fund Postdoctoral Fellow at Harvard University. Since 1994, he has received over $20 million in research funding and has published over 130 technical articles and two books on his research. His research group is engaged in developing fundamental and applied research in the area of microfluidics, point-of-care (POC) diagnostic devices, fuel cells, and chemical and biochemical separations. He has mentored over 130 undergraduate, masters, and high school students and 12 postdoctoral fellows and visiting scientists in his laboratories resulting in over 185 student presentations. He is the director of the NSF-funded Cal State LA-Penn State U Partnerships for Research and Education in Materials (PREM) program, co-PI of the NSF CREST Center for Energy and Sustainability. and PI of a W. M Keck Foundation grant for course-based undergraduate research experiences (CUREs).
In 1997 he was awarded a NSF CAREER Award and in 2007 he received the CSUPERB Biotechnology Faculty Research Award. Gomez sat on the board of directors (1989-1991, 1993-1996, 1997-2003) and was secretary (1997-2003) of the Society for Advancement of Chicanos and Native Americans in Science (SACNAS). In 2003 he received the Undergraduate Institution Mentor Award from SACNAS. In 1993 he received the Hispanic Engineer National Achievement Award Conference (HENAAC) Most Aspiring Scientist Award. He has served on a number of NSF, NIH, and NRC review panels. He is strongly committed to science education having served on a number of community and university educational policy and scholarship committees. Gomez was a member of the Montebello Unified School District (MUSD) Board of Education from 1997-2001 and in 2002 was named Democrat of the Year for the 58th Assembly District. In 1998 he received the Outstanding Young Educator Award from the California Jaycees. Gomez served on the Montebello City Council from 2009-2013 and was Mayor from 2011-2012. He currently serves on the Board of Directors of the Southern California Conferences for Undergraduate Research (SCCUR) and on the Leo Buscaglia Foundation Board of Directors.
We thank the following for their gracious support.
Undergraduate Students
Ashley Reyes Ana Villalpando
Biochemistry Chemistry
Alyssa Wong
Biology
Visiting Research Scientist
Dr. Gomez's Alumni
- Dong S. Zhao, M.S. 1997, Ph.D. from UC Riverside.
- Eun-Soo Kwak, M.S. 1997, Ph.D. from UT, Austin.
- Jane Kawaoka, B.S. 1998, Ph.D. from Columbia University.
- Chuauthemoc Arellanes, B.S. 1998, Ph.D. from UCLA.
- Sally Esquivel, B.S. 1998, D.D.S. from UCLA, practicing dentist in SoCal.
- Erica Mito, B.S., 2001, in industry.
- John Kaddis, B.S., 2001, Ph.D. from USC, assistant professor at City of Hope.
- Joseph Heintz, B.S. 2001, working at Coca-Cola.
- Alfredo Plazas, B.S. 2001, elementary school teacher.
- Cynthia Kodama, B.S. 2002, D.O. from Western University of Health Sciences, practicing physician in SoCal.
- Catherine Silverio, M.S., 2002, Ph.D. from UCLA.
- Maryam Azad, M.S., 2003, in industry.
- Valerie Villareal, B.S., 2003, Ph.D. from UCLA, in industry.
- Dinora Chinchilla, B.S., 2005, M.D. from UC Irvine, practicing physician in SoCal.
- Jose Zavaleta, M.S., 2005, in industry.
- Jerry Fields, M.S., 2005, in industry.
- Taguhi Sogomonyan, B.S. 2007, in industry.
- Abby Brown, B.S. 2005, in industry.
- Froseen Dahdouh, B.A., 2006, working for the government.
- Dr. Attila Gaspar, Visiting Scientist, 2006-2007, Professor of Chemistry, University of Debrecen, Hungary.
- Ruthy Montes, M.S., 2007, M.D. from USC, practicing physician in SoCal.
- Alvaro Gomez, B.S., 2007, in industry.
- Lilia Hernandez, M.S. 2007, in industry.
- Violet Calderon, B.S., 2008, in industry.
- Maral Sarikhani, M.S., 2008, Ph.D. from the University of Pisa.
- Schetema Stevens, M.S., 2008, Ph.D. from UNLV.
- Mark Goldberg, M.S., 2008, Ph.D. from Caltech
- Dr. Xiaojun Liu, postdoc 2007-2009, in industry.
- Dr. Roger Lo, postdoc 2008-2009, Associate Professor of Chemical Engineering, CSU Long Beach.
- Alejandra Ramirez, M.S., 2009, working for the government.
- Erica Garcia, M.S., 2009, M.S. from Caltech, working at Caltech in grants administration.
- Toni Ann Riveros, 2010, M.D. from UCLA; resident in Washington State.
- Dan Ted Botoaca, M.S., 2012.
- Marisol Salgado, B.S. 2012, in industry.
- Amy Wat, B.S., 2012, Ph.D. from UC Berkeley.
- Judith Alvarado, M.S., 2012, Ph.D. from UC San Diego.
- Juliette Ohan, B.S., 2013, M.S. from Oregon State University.
- Hector Carmona, B.S., 2013, D.D.S. from UC San Francisco, resident in Louisiana.
- Micah Eropkin, B.S., 2014, M.S. from UC Riverside.
- Lenny Sanchez, M.S., 2014, in industry.
- Mary Arrastia, B.S., 2015, Ph.D. from Caltech.
- Maria Ortega, M.S., 2015, in industry.
- Chris Darakjian, M.S., 2015, high school teacher.
- Vicente Galvan, B.S., 2016, in the Ph.D. program at USC.
- Kryls Domalaon, B.S., 2016, in medical school at UC Davis.
- Lissette Estala, B.S., 2016, M.S. from USC, in medical school at the University of Arizona.
- Ani Avoundjian, B.S., 2017, Pharm. D. from USC.
- Santino Valiulis, B.S., 2017, in the Ph.D. program at UC Riverside.
- Catherine Tang, B.S., 2017, at LADWP.
- Alex Mendez, B.S., 2017, in industry.
- Dr. Chunye Liu, Visiting Scientist, 2016-2017, Associate Professor of Pharmacy, Xi'an Medical University, China.
- Michelle Gaines, B.S., 2018, in industry.
- Ariana Gonzalez, B.S., 2018, in medical school at UCLA.
- Paolo Argelles, B.S., 2018, M.S. from Cornell University.
- Franky Bernal, B.S., 2018, in the Ph.D. program at UC Berkeley.
- Grenalynn Ilacas, M.S., 2018, in the Ph.D. program at SUNY Stonybrook.
- Wilson Lee, B.S., 2018, in the Ph.D. program at USC.
- Dr. Maria Jose Gonzalez Guerrero, postdoc, 2016-2018, in industry, Barcelona, Spain.
- Alexis Basa, B.S., 2019, D.O. school.
- Natalia Neris, M.S., 2019, in the Ph.D. program at UC Santa Barbara.
- Samantha Burrola, B.S., 2019, in pharmacy school at USC.
- Ricardo Guevara, B.S., 2019, in D.O. school.
- Ricardo Ortiz, B.S., 2019, in industry.
- Kathryn Uchida, B.S., 2020, UC Irvine medical school.
- Jessica Vazquez, B.S., 2020, in M.S. program at Cal State LA.
- Jennifer Galvez, B.S., 2020.
- Lauren Duenas, B.S., 2020, in the Ph.D. program at Penn State University.
Paper Microfluidics
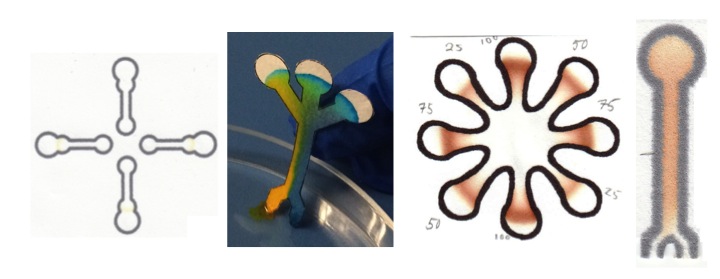
Microfluidic paper analytical devices (µPADs) have great potential as a platform in point-of-care (POC) diagnostic devices and fuel cells. Major reasons for their attractiveness as a substrate for microfluidic devices (MDs) include low cost, compatibility with a plethora of chemical/biochemical reagents and, ability to wick aqueous fluidics without the use of active pumping. Furthermore, paper is thin, available in a variety of thicknesses, lightweight, easy to stack, store, and transport, is compatible with biological samples given its composition (cellulose or blends thereof), easy to chemically modify for functionalization, typically white thereby amenable for colorimetric tests, and is available in many forms with a diverse range of properties. Paper microfluidics is emerging as a multiplexable POC platform that may transcend the capabilities of current assays in settings where resources are limited. We have examined a number of model biochemical systems and have developed a number of new techniques employing µPADs. In addition to further developing new methodologies and examining other biological systems we are also using µPADs in the analysis of environmental and forensic problems. Figure 1 shows representative µPADs developed in our labs.
Figure 1. Representative paper microfluidic chips.
References:
- “Paper-Based Microfluidic Point-of-Care Diagnostic Devices for Monitoring Drug Metabolism,” Chong, H.; Koo, Y.; Collins, B.; Gomez, F. A. Sankar, J.; Yun, Y. J. Nanomedic. Biotherapeu. Discovery 2013, 3, e122.
- Paper Microfluidic-Based Enzyme Catalyzed Double Microreactor”, Ferrer, I. M.; Valadez, H.; Estala, L.; Gomez, F. A. Electrophoresis, 2014, 35, 2417-2419.
- “Paper Microfluidics in Bioanalysis,” Gomez, F. A. Bioanalysis, 2014, 6, 2911-2914.
- “Development of a Microfluidic-Based Assay on a Novel Nitrocellulose Platform”, Arrastia, M.; Avoundjian, A.; Ehrlich, P. S.; Eropkin, M.; Levine, L.; Gomez, F. A. Electrophoresis, 2015, 36, 884-888.
- “A Microfluidic Direct Formate Fuel Cell on Paper”, Copenhaver, T. S.; Purohit, K. H.; Domalaon, K.; Pham, L.; Burgess, B. J.; Manorothkul, N.; Galvan, V., Sotez, S.; Gomez, F. A.; Haan, J. L. Electrophoresis, 2015, 36, 1825-1829.
- “An Improved Alkaline Direct Formate Paper Microfluidic Fuel Cell”, Galvan, V.; Domalaon, K.; Tang, C.; Sotez, S.; Mendez, A.; Jalali-Heravi, M.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F. A. Electrophoresis, 2016, 37, 504-510.
- “Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays”, Gallibu, C.; Gallibu, C.; Avoundjian, A.; Jalali-Heravi, M.; Gomez, F. A. Micromachines, 2016, 7, 6-9.
- “A Microfluidic Galvanic Cell on a Single Layer of Paper”, Purohit, K. H.; Emrani, S.; Rodriguez, S.; Liaw, S. –S.; Galvan, V.; Domalaon, K.; Gomez, F. A.; Haan, J. L. J. Power Sources, 2016, 318, 163-169.
- “Mixed Thread/Paper-Based Microfluidic Chips as a Platform for Glucose Assays,” Gonzalez, A.; Estala, L.; Gaines, M.; Gomez, F. A. Electrophoresis, 2016, 37, 1685-1690.
- “Use of a Computational Model to Optimize a Glucose Assay on a Paper Microfluidic Platform”, Avoundjian, A.; Jalali-Heravi, M.; Gomez, F. A. Anal. Bioanal. Chem. 2017, 409, 2697-2703.
- “Experimental Analysis of Fabrication Parameters in the Development of Microfluidic Paper-Based Analytical Devices (uPADS)” Lee, W.; Gomez, F. A. Micromachines, 2017, 8, 99.
- “A Microfluidic Paper-Based Device to Assess Acetylcholinesterase Activity”, Liu, C.; Gomez, F. A. Electrophoresis, 2017, 38, 1002-1006.
- “Paper-Based Point-of-Care Testing in Disease Diagnostics”, Syedmoradi, L.; Gomez, F. A. Bioanalysis, 2017, 9, 841-843.
- “An Inexpensive Paper-Based Aluminum Air Battery”, Avoundjian, A.; Galvan, V.; Gomez, F. A. Micromachines, 2017, 8 222.
- “Enzyme Chemotaxis on Paper-Based Devices”, Ilacas, G. C.; Basa, A.; Sen, A.; Gomez, F. A. Anal. Sci. 2018, 34, 115-119.
- “Thread/Paper- and Paper-Based Microfluidic Devices for Glucose Assays Employing Artificial Neural Networks”, Lee, W.; Gonzalez, A.; Arguelles, P. N.; Guevara, R.; Gonzalez-Guerrero, M. J.; Gomez, F. A. Electrophoresis, 2018, 12, 1443-1451.
- “Simple and Sensitive Colorimetric Assay System for Dopamine Using Microfluidic Paper-Based Analytical Devices”, Liu, C.; Gomez, F. A.; Miao, Y.; Cui, P.; Lee, W. Talanta 2019, 194, 171-176.
- “3D Multilayered Paper- and Thread/Paper-Based Microfluidic Devices for Bioassays”, Neris, N. M.; Guevara, R. D.; Gonzalez, A.; Gomez, F. A. Electrophoresis, 2019, 40, 296-303.
- “Microfluidic Paper-Based Analytical Devices (uPADs): Miniaturization and Enzyme Storage Studies”, Ilacas, G. C.; Gomez, F. A. Anal. Sci. 2019, 35, 379-384.
Thread Microfluidics
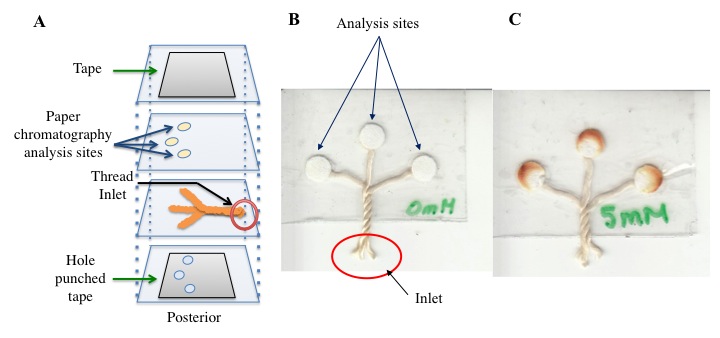
Microfluidics-based technologies continue to gain traction as alternatives to traditional techniques and have great potential in resource-limited settings where access to more expensive instrumentation is not always possible. Recently, thread has been found to be a useful matrix for the fabrication of biomedical devices. It has a number of characteristics that make it a viable material as a microfluidic platform: 1) It is inexpensive, widely used, and is readily available. 2) It is lightweight, flexible, and difficult to break. 3) It is usually hydrophilic material or can be made hydrophilic by techniques such as plasma oxidation. 4) Thread can be manipulated easily since it can be knitted or woven. Furthermore, since it can be manipulated this means liquid transport can also be manipulated. 5) It can be functionalized using well-known chemical procedures. We have developed a number of novel microfluidic thread-based analytical devices (µTADs) and thread/paper-based analytical devices (µTPADs) to detect a number of analytes through colorimetric assays using commercially available nylon thread. We feel these devices have the potential for use in healthcare and other industries in both developed and underdeveloped resource-limited regions. Figure 2 is a recently developed glucose assay using thread microfluidics.
Figure 2. (A) Schematic representation of the µTPAD device fabrication process. The arrows noted in the figure reference the location of a labeled component. (B) Representative nylon flat µTPAD. (C) Photograph of 5 mM concentration of glucose in the nylon flat µTPAD.
References:
- “Mixed Thread/Paper-Based Microfluidic Chips as a Platform for Glucose Assays,” Gonzalez, A.; Estala, L.; Gaines, M.; Gomez, F. A. Electrophoresis, 2016, 37, 1685-1690.
- “Thread-Based Microfluidic Chips as a Platform to Assess Acetylcholinesterase Activity”, Gonzalez, A.; Gaines, M.; Gomez, F. A. Electrophoresis, 2017, 38, 996-1001.
- “Enzyme-Linked Immunosorbent Assays (ELISA) Based on Thread, Paper, and Fabric”, Gonzalez, A.; Gaines, M.; Gallegos, L. Y.; Guevara, R.; Gomez, F. A. Electrophoresis, 2018, 39, 476-484.
- “Thread/Paper- and Paper-Based Microfluidic Devices for Glucose Assays Employing Artificial Neural Networks”, Lee, W.; Gonzalez, A.; Arguelles, P. N.; Guevara, R.; Gonzalez-Guerrero, M. J.; Gomez, F. A. Electrophoresis, 2018, 12, 1443-1451.
- “A Microfluidic Glucose Sensor Incorporating a Novel Thread-Based Electrode System”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 2131-2135.
- “Microfluidic Thread-Based Electrode System to Detect Glucose and Acetylthiocholine”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 3082-3086.
- “3D Multilayered Paper- and Thread/Paper-Based Microfluidic Devices for Bioassays”, Neris, N. M.; Guevara, R. D.; Gonzalez, A.; Gomez, F. A. Electrophoresis, 2019, 40, 296-303.
Microfluidic Fuel Cells and Batteries
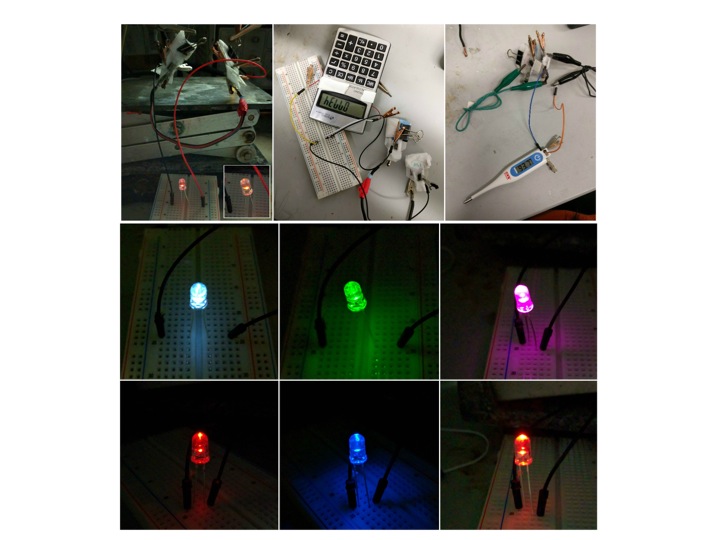
There is a great need to develop efficient and small power sources to operate portable electronic devices. Over the past several years, we have been focused on developing microfluidic fuel cells (MFCs) utilizing methanol, formic acid, and hydrogen. The use of these fuels entails one of the most promising mobile technologies by which such power can be provided. FCs can be considered chemical reactors designed to convert chemical reactant streams into electrical energy and chemical products. Our work is divided between paper MFCs and those built on a poly(dimethylsiloxane) (PDMS) platform. Focus is on optimizing the design of the FCs, catalyst and its loading, including the type of membrane to use, the catalyst loading, the mechanism of catalyst loading onto the membrane, to hot press or not the catalyst, and variations in the design. FIgure 3 displays some of our representative work.
Figure 3. Microfluidic fuel cells (MFCs), small devices, and light emitting diodes (LEDs) powered by MFCs.
References:
- “A Microfluidic Direct Formate Fuel Cell on Paper”, Copenhaver, T. S.; Purohit, K. H.; Domalaon, K.; Pham, L.; Burgess, B. J.; Manorothkul, N.; Galvan, V., Sotez, S.; Gomez, F. A.; Haan, J. L. Electrophoresis, 2015, 36, 1825-1829.
- “An Improved Alkaline Direct Formate Paper Microfluidic Fuel Cell”, Galvan, V.; Domalaon, K.; Tang, C.; Sotez, S.; Mendez, A.; Jalali-Heravi, M.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F. A. Electrophoresis, 2016, 37, 504-510.
- “A Microfluidic Galvanic Cell on a Single Layer of Paper”, Purohit, K. H.; Emrani, S.; Rodriguez, S.; Liaw, S. –S.; Galvan, V.; Domalaon, K.; Gomez, F. A.; Haan, J. L. J. Power Sources, 2016, 318, 163-169.
- “Fabric-Based Alkaline Direct Formate Microfluidic Fuel Cells”, Domalaon, K.; Tang, C.; Mendez, A.; Bernal, F.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F. A. Electrophoresis, 2017, 38, 1224-1231.
- “An Inexpensive Paper-Based Aluminum Air Battery”, Avoundjian, A.; Galvan, V.; Gomez, F. A. Micromachines, 2017, 8 222.
- “Miniaturized Al/AgO Coin Shape and Self-Powered Battery Featuring Painted Paper Electrodes for Portable Applications”, Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2018, 273, 101-107.
- “An Optimized Microfluidic Paper-Based NiOOH/Zn Alkaline Battery”, Burrola, S.; Gonzalez Guerrero, M. J.; Avoundjian, A.; Gomez, F. A. Electrophoresis, 2019, 40, 469-472.
- "An All-Printed 3D-Zn/Fe3O4 Paper Battery", Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2019, 289, 226-233.
Integration of Modeling in the Optimization of Experimental Parameters in Microfluidics and Capillary Electrophoresis
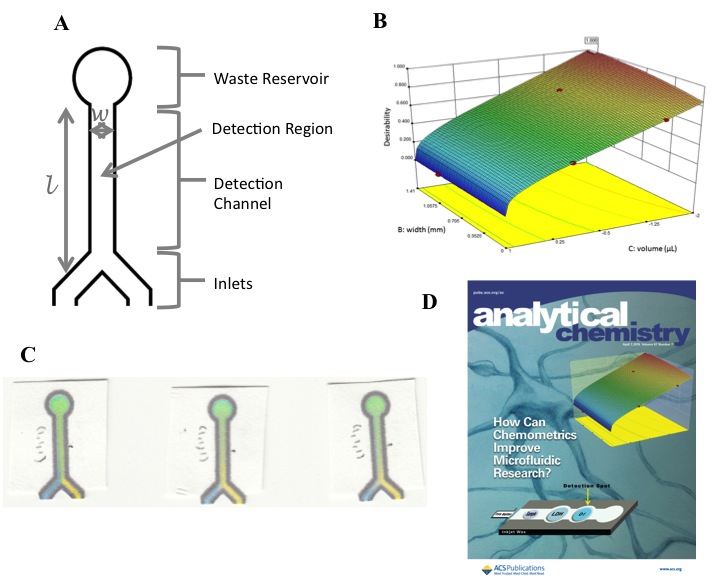
For several years we have focused and collaborated in applying chemometrics in the optimization of experimental parameters in microfluidics and capillary electrophoresis (CE). The heterogeneity of complex chemical and biological systems raises fundamental difficulties with regard to the ability of investigators to separate and detect analytes of interest, and to correlate variables and predict patterns within samples and differing sample matrices. Fortunately, the recent development of novel learning algorithms, hybrid computational techniques, and gains in raw computing power and speed, has raised several fundamental modeling and simulation research lines to account for these difficulties. Previous work has applied experimental design-based techniques in flow-injection, affinity capillary electrophoresis (ACE) and electrophoretically mediated microanalysis (EMMA). Figure 4 shows some of our recent work.
Figure 4. (A) Schematic representation of the paper microfluidic chip. (B) Three-dimensional response surface for channel width and sample volume. (C) Examples of chips after mixing with blue and yellow dyes. (D) Anal. Chem. cover from Jalali-Heravi, M.; Arrastia, M.; Gomez, F. A. Anal. Chem. 2015, 87, 3544-3555.
References:
-
"Implementation of Chemometric Methodology in Affinity Capillary Electrophoresis (ACE): Predictive Investigation of Protein-Ligand Binding", Hanrahan, G.; Montes, R.; Pao, A.; Johnson, A.; Gomez, F. A. Electrophoresis, 2007, 28, 2853-2860.
-
"Response Surface Examination of the Relationship Between Experimental Conditions and Product Distribution in Electrophoretically Mediated Microanalysis (EMMA)," Montes, R.; Gomez, F. A.; Hanrahan, G., Electrophoresis, 2008, 29, 375-380.
-
"Chemometric Experimental Design-Based Optimization Techniques in Capillary Electrophoresis: A Critical Review of Modern Applications", Hanrahan, G.; Montes, R.; Gomez, F. A., Anal. Bioanal. Chem. 2008, 390, 169-179.
-
"Chemometrical Experimental Design-Based Optimization Studies in Capillary Electrophoresis Applications", Montes, R.; Dahdouh, F.; Riveros, T. A.; Hanrahan, G.; Gomez, F. A. LCGC, 2008, 26, 712-721.
-
"Use of Chemometric Methodology in Optimizing Conditions for Competitive Binding Partial Filling Affinity Capillary Electrophoresis (PFACE)", Montes, R.; Hanrahan, G.; Gomez, F. A., Electrophoresis, 2008, 29, 3325-3332.
-
"Chemometrical Examination of Active Parameters and Interactions in Flow Injection-Capillary Electrophoresis (FI-CE)," Dahdouh, F. T.; Clarke, K.; Salgado, M.; Hanrahan, G.; Gomez, F. A. Electrophoresis, 2008, 29, 3779-3785.
-
"Application of Artificial Neural Networks in the Prediction of Product Distribution in Electrophoretically Mediated Microanalysis (EMMA)", Riveros, T. A.; Porcasi,L.; Muliadi, S.; Hanrahan, G.; Gomez, F. A. Electrophoresis 2009, 30, 2385-2389.
-
"On-Capillary Derivatization Using a Hybrid Artificial Neural Network-Genetic Algorithm Approach", Riveros, T. A.; Hanrahan, G.; Muliadi, S.; Arceo, J.; Gomez, F. A. Analyst, 2009, 134, 2067-2070.
-
"Implementation of a Genetically Tuned Neural Platform in Optimizing Fluorescence from Receptor-Ligand Binding Interactions on Microchips", Alvarado, J.; Hanrahan, G.; Nguyen, H. T. H.; Gomez, F. A. Electrophoresis, 2012, 33, 2711-2717.
-
"Application of a Computational Neural Network to Optimize the Fluorescence from a Receptor-Ligand Interaction on a Microfluidic Chip", Ortega, M.; Hanrahan, G.; Arceo, M.; Gomez, F. A. Electrophoresis, 2015, 36, 393-397.
-
“How Chemometrics can Improve Microfluidic Research?”, Jalali-Heravi, M.; Arrastia, M.; Gomez, F. A. Anal. Chem. 2015, 87, 3544-3555 (cover).
-
“Use of a Computational Model to Optimize a Glucose Assay on a Paper Microfluidic Platform”, Avoundjian, A.; Jalali-Heravi, M.; Gomez, F. A. Anal. Bioanal. Chem. 2017, 409, 2697-2703.
-
“Thread/Paper- and Paper-Based Microfluidic Devices for Glucose Assays Employing Artificial Neural Networks”, Lee, W.; Gonzalez, A.; Arguelles, P. N.; Guevara, R.; Gonzalez-Guerrero, M. J.; Gomez, F. A. Electrophoresis, 2018, 12, 1443-1451
Publications
(Underlined names denote undergraduate student co-authors.)
126. "Thread- and Capillary Tube-Based Electrodes for the Detection of Glucose and Acetylthiocholine", Uchida, K.; Duenas, L.; Gomez, F. A. Micromachines, 2020, 11, 920, https://doi/org/10.3390/mi11100920.
125. “Production of a NiO/Al Primary Battery Employing Powder-Based Electrodes”, Burrola, S.; Horii, M.; Gonzalez-Guerrero, M. J.; Bachman, J. C.; Gomez, F. A. Electrophoresis, 2020, 41, 131-136.
124. “Microfluidic Paper-Based Analytical Devices (uPADs): Miniaturization and Enzyme Storage Studies”, Ilacas, G. C.; Gomez, F. A. Anal. Sci. 2019, 35, 379-384.
123. "An All-Printed 3D-Zn/Fe3O4 Paper Battery", Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2019, 289, 226-233.
122. “Paper-Based Microfluidic Devices for Glucose Assays Employing a Metal-Organic Framework (MOF)”, Ilacas, G. C.; Basa, A.; Nelms, K.; Sosa, J.; Liu, Y.; Gomez, F. A. Anal. Chim. Acta, 2019, 1055, 74-80.
121. “An Optimized Microfluidic Paper-Based NiOOH/Zn Alkaline Battery”, Burrola, S.; Gonzalez Guerrero, M. J.; Avoundjian, A.; Gomez, F. A. Electrophoresis, 2019, 40, 469-472.
120. “3D Multilayered Paper- and Thread/Paper-Based Microfluidic Devices for Bioassays”, Neris, N. M.; Guevara, R. D.; Gonzalez, A.; Gomez, F. A. Electrophoresis, 2019, 40, 296-303.
119. “Simple and Sensitive Colorimetric Assay System for Dopamine Using Microfluidic Paper-Based Analytical Devices”, Liu, C.; Gomez, F. A.; Miao, Y.; Cui, P.; Lee, W. Talanta 2019, 194, 171-176.
118. “Microfluidic Thread-Based Electrode System to Detect Glucose and Acetylthiocholine”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 3082-3086.
117. “A Microfluidic Glucose Sensor Incorporating a Novel Thread-Based Electrode System”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 2131-2135.
116. “Thread/Paper-, and Fabric Enzyme-Linked Immunosorbent Assays (ELISA)”, Gonzalez, A.; Gaines, M.; Gallegos, L. Y.; Guevara, R.; Gomez, F. A. Methods, 2018, 146, 58-65.
115. “Thread/Paper- and Paper-Based Microfluidic Devices for Glucose Assays Employing Artificial Neural Networks”, Lee, W.; Gonzalez, A.; Arguelles, P. N.; Guevara, R.; Gonzalez-Guerrero, M. J.; Gomez, F. A. Electrophoresis, 2018, 12, 1443-1451.
114. “Miniaturized Al/AgO Coin Shape and Self-Powered Battery Featuring Painted Paper Electrodes for Portable Applications”, Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2018, 273, 101-107.
113. “Enzyme Chemotaxis on Paper-Based Devices”, Ilacas, G. C.; Basa, A.; Sen, A.; Gomez, F. A. Anal. Sci. 2018, 34, 115-119.
112. “Enzyme-Linked Immunosorbent Assays (ELISA) Based on Thread, Paper, and Fabric”, Gonzalez, A.; Gaines, M.; Gallegos, L. Y.; Guevara, R.; Gomez, F. A. Electrophoresis, 2018, 39, 476-484.
111. “An Inexpensive Paper-Based Aluminum Air Battery”, Avoundjian, A.; Galvan, V.; Purohkit, K.; Haan, J.; Gomez, F. A. Micromachines, 2017, 8, 222.
110. “Paper-Based Point-of-Care Testing in Disease Diagnostics”, Syedmoradi, L.; Gomez, F. A. Bioanalysis, 2017, 9, 841-843.
109. “Fabric-Based Alkaline Direct Formate Microfluidic Fuel Cells”, Domalaon, K.; Tang, C.; Mendez, A.; Bernal, F.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F. A. Electrophoresis, 2017, 38, 1224-1231.
108. “A Microfluidic Paper-Based Device to Assess Acetylcholinesterase Activity”, Liu, C.; Gomez, F. A. Electrophoresis, 2017, 38, 1002-1006.
107. “Thread-Based Microfluidic Chips as a Platform to Assess Acetylcholinesterase Activity”, Gonzalez, A.; Gaines, M.; Gomez, F. A. Electrophoresis, 2017, 38, 996-1001.
106. “Use of a Computational Model to Optimize a Glucose Assay on a Paper Microfluidic Platform”, Avoundjian, A.; Jalali-Heravi, M.; Gomez, F. A. Anal. Bioanal. Chem. 2017, 409, 2697-2703.
105. “Experimental Analysis of Fabrication Parameters in the Development of Microfluidic Paper-Based Analytical Devices (mPADS)” Lee, W.; Gomez, F. A. Micromachines, 2017, 8, 99.
104. “Point of Care Testing: The Impact of Nanotechnology”, Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F. A.; Hajghassem, H.; Omidfar, K. Biosens. Bioelectron. 2017, 87, 373-387.
103. “Mixed Thread/Paper-Based Microfluidic Chips as a Platform for Glucose Assays,” Gonzalez, A.; Estala, L.; Gaines, M.; Gomez, F. A. Electrophoresis, 2016, 37, 1685-1690.
102. “Microscale Bioanalysis,” Knutsson, M.; Timmerman, P.; Gomez, F. A. Bioanalysis, 2016, 8, 859-862.
101. “A Microfluidic Galvanic Cell on a Single Layer of Paper”, Purohit, K. H.; Emrani, S.; Rodriguez, S.; Liaw, S. –S.; Galvan, V.; Domalaon, K.; Gomez, F. A.; Haan, J. L. J. Power Sources, 2016, 318, 163-169.
100. “Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays”, Gallibu, C.; Gallibu, C.; Avoundjian, A.; Gomez, F. A. Micromachines, 2016, 7, 6-9.
99. “An Improved Alkaline Direct Formate Paper Microfluidic Fuel Cell”, Galvan, V.; Domalaon, K.; Tang, C.; Sotez, S.; Mendez, A.; Jalali-Heravi, M.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F. A. Electrophoresis, 2016, 37, 504-510.
98. “A Microfluidic Direct Formate Fuel Cell on Paper”, Copenhaver, T. S.; Purohit, K. H.; Domalaon, K.; Pham, L.; Burgess, B. J.; Manorothkul, N.; Galvan, V., Sotez, S.; Gomez, F. A.; Haan, J. L. Electrophoresis, 2015, 36, 1825-1829.
97. “How Can Chemometrics Improve Microfluidic Research?”, Jalali-Heravi, M.; Arrastia, M.; Gomez, F. A. Anal. Chem. 2015, 87, 3544-3555 (cover).
96. “Development of a Microfluidic-Based Assay on a Novel Nitrocellulose Platform”, Arrastia, M.; Avoundjian, A.; Ehrlich, P. S.; Eropkin, M.; Levine, L.; Gomez, F. A. Electrophoresis, 2015, 36, 884-888.
95. “Application of a Computational Neural Network to Optimize the Fluorescence from a Receptor-Ligand Interaction on a Microfluidic Chip”, Ortega, M.; Hanrahan, G.; Arceo, M.; Gomez, F. A. Electrophoresis, 2015, 36, 393-397.
94. “Development of Microfluidic-Based Assays to Estimate the Binding between Osteocalcin and Fluorescent Antibodies,” Carmona, H.; Valadez, H.; Yun, Y.; Estala, L.; Arrastia, M.; Gomez, F. A. Talanta, 2015, 132, 676-679.
93. “Paper Microfluidics in Bioanalysis,” Gomez, F. A. Bioanalysis, 2014, 6, 2911-2914.
92. “Paper Microfluidic-Based Enzyme Catalyzed Double Microreactor”, Ferrer, I. M.; Valadez, H.; Estala, L.; Gomez, F. A. Electrophoresis, 2014, 35, 2417-2419.
91. “Paper-Based Microfluidic Point-of-Care Diagnostic Devices for Monitoring Drug Metabolism,” Chong, H.; Koo, Y.; Collins, B.; Gomez, F. A. Sankar, J.; Yun, Y. J. Nanomedic. Biotherapeu. Discovery 2013, 3, e122.
90. “Use of Surface Plasmon Resonance to Study the Adsorption of Detergents on Poly(dimethysiloxane) Surfaces”, Gaspar, A.; Kecskemeti, A.; Gomez, F. A. Electrophoresis, 2013, 34, 1249-1252.
89. “Application of Surface Plasmon Resonance Spectroscopy for Adsorption Studies of Different Types of Components on Poly(dimethylsiloxane)”, Gaspar, A.; Gomez, F. A. Anal. Chim. Acta, 2013, 777, 72-77.
88. “The Future of Microfluidic Point-of-Care (POC) Diagnostic Devices,” Gomez, F. A. Bioanalysis, 2013, 5, 1-3.
87. “Implementation of a Genetically Tuned Neural Platform in Optimizing Fluorescence from Receptor-Ligand Binding Interactions on Microchips,” Alvarado, J.; Hanrahan, G.; Nguyen, H. T. H.; Gomez, F. A. Electrophoresis, 2012, 33, 2711-2717.
86. “Glass/PDMS Hybrid Microfluidic Device Integrating Vertically Aligned SWCNTs for Electrochemical Determination,” Moraes, F.; Lima, R.; Segato, T.; Cesarino, T.; Melendez, J.; Machado, S.; Gomez, F. A.; Carrilho, E. Lab Chip, 2012, 12, 1959-1962.
85. “Development of an Ultra-Low Volume Flow-Cell for Surface Plasmon Resonance Detection in a Miniaturized Capillary Electrophoresis System,” Gaspar, A.; Gomez, F. A. Electrophoresis, 2012, 33, 1723-1728.
84. “Human-on-a-Chip Technologies as the Next Generation Drug Screening Platforms,” Yun, Y.; Lee, S.; Collins, B.; Gomez, F. A. Sankar, J. J. Nanomedic. Biotherapeu. Discovery 2012, 2, 1000e113.
83. “Facile Fabrication of an Interface for On-Line Coupling of Microchip Capillary Electrophoresis to Surface Plasmon Resonance,” Liu, X.; Du, M.; Zhou, F.; Gomez, F. A. Bioanalysis 2012, 4, 373-379.
82. "Split Injection: A Simple Introduction of Subnanoliter Sample Volumes for Chip Electrophoresis,” Gáspár, A.; Koczka, P. I.; Carmona, H.; Gomez, F. A. Microchemical J. 2011, 99, 180-185.
81. “Microfluidic Thin Chips for Chemical Separations,” Gaspar, A.; Salgado, M.; Stevens, S.; Gomez, F. A. Electrophoresis 2010, 31, 2520-2525.
80. “Analysis and Stability Study of Temozolomide Using Capillary Electrophoresis”, Andrasi, M.; Bustos, R.; Gaspar, A.; Gomez, F. A.; Kelkner, A. J. Chromatogr. B, 2010, 878, 1801-1808.
79. “Use of Magnetic Beads in Microfluidic Binding Assays and On-Chip Enzymatic Microreactions,” Riveros, T. A.; Lo, R.; Liu, X.; Valdez, A.; Lozano, M.; Gomez, F. A. Am. Lab. 2010, 42, 11-19.
78. “On-Capillary Derivatization Using a Hybrid Artificial Neural Network-Genetic Algorithm Approach,” Riveros, T. A.; Hanrahan, G.; Muliadi, S.; Arceo, J.; Gomez, F. A. Analyst, 2009, 134, 2067-2070.
77. “Application of Artificial Neural Networks in the Prediction of Product Distribution in Electrophoretically Mediated Microanalysis (EMMA),” Riveros, T. A.; Porcasi,L.; Muliadi, S.; Hanrahan, G.; Gomez, F. A. Electrophoresis 2009, 30, 2385-2389.
76. “Application of External Micro-Spectrophotometric Detection for Microchips”, Gaspar, A.; Bacsi, I.; Garcia, E. F.; Braun, M.; Gomez, F. A. Anal. Bioanal. Chem. 2009, 395, 473-478.
75. “Use of Magnetic Beads to Study the Interaction of Ristocetin with Peptides and Bacteria,” Sarakhanikhorami, M.; Lo, R. C.; Gomez, F. A. Bioanalysis, 2009, 1, 715-719.
74. “Fabrication of a Microfluidic Enzyme Reactor Utilizing Magnetic Beads,” Liu, X.; Lo, R.; Gomez, F. A. Electrophoresis, 2009, 30, 2129-2133.
73. "Development of Microfluidic Chips for Heterogeneous Receptor-Ligand Interaction Studies,” Goldberg, M. D.; Lo, R. C.; Abele, S.; Macka, M.; Gomez, F. A. Anal. Chem. 2009, 81, 5095-5098.
72. “Microchip Frontal Affinity Chromatography to Study the Binding of a Ligand to Teicoplanin-Derivatized Microbeads”, Liu, X.; Gomez, F. A. Electrophoresis 2009, 30, 1194-1197.
71. “Recent Advances in Affinity Capillary Electrophoresis (2007),” Liu, X.; Dahdouh, F.; Salgado, M.; Gomez, F. A. J. Pharm. Sci. 2009, 98, 394-410.
70. “Frontal Analysis Microchip Capillary Electrophoresis to Study the Binding of Ligands to Receptors Derivatized on Magnetic Beads,” Liu, X.; Gomez, F. A. Anal. Bioanal. Chem. 2009, 393, 615-621.
69. “Microfluidic Polymerase Chain Reaction,” Maltezos, G. M.; Gomez, A.; Zhong, J.; Gomez, F. A.; Scherer, A. Appl. Phys. Lett. 2008, 93, 243901.
68. “Magnetic Microsphere-Based Methods to Study the Interaction of Teicoplanin with Peptides and Bacteria,” Piyasena, M. E.; Real, L. J.; Diamond, R. A.; Xu, H.; Gomez, F. A. Anal. Bioanal. Chem,, 2008, 392, 877-886.
67. “Fritless Chromatographic Microfluidic-Based Columns for Chemical Separations,” Gaspar, A.; Goldberg, M.; Baghdachi, S.; Stevens, S.; Torres, J.; Salgado, M.; Gomez, F. A. Am. Lab. 2008, 40, 13-16.
66. “Chemometrical Examination of Active Parameters and Interactions in Flow Injection-Capillary Electrophoresis (FI-CE),” Dahdouh, F. T.; Clarke, K.; Salgado, M.; Hanrahan, G.; Gomez, F. A. Electrophoresis, 2008, 29, 3779-3785.
65. “Use of Chemometric Methodology in Optimizing Conditions for Competitive Binding Partial Filling Affinity Capillary Electrophoresis (PFACE)”, Montes, R.; Hanrahan, G.; Gomez, F. A., Electrophoresis, 2008, 29, 3325-3332.
64. “Chemometrical Experimental Design-Based Optimization Studies in Capillary Electrophoresis Applications”, Montes, R.; Dahdouh, F.; Riveros, T. A.; Hanrahan, G.; Gomez, F. A. LCGC, 2008, 26, 712-721.
63. “Magnetically Controlled Valve for Flow Manipulation in Polymer Microfluidic Devices,” Gaspar, A.; Piyasena, M.; Daroczi, L.; Gomez, F. A. Micro. Nano. 2008, 6, 525-531.
62. “Electrochromatography in Fritless Chromatographic Microchips Packed with Conventional Reversed-Phase Silica Particles”, Gaspar, A.; Hernandez, L.; Stevens, S.; Gomez, F. A., Electrophoresis, 2008, 29, 1638-1642.
61. “Chemometric Experimental Design-Based Optimization Techniques in Capillary Electrophoresis: A Critical Review of Modern Applications”, Hanrahan, G.; Montes, R.; Gomez, F. A., Anal. Bioanal. Chem. 2008, 390, 169-179.
60. “Response Surface Examination of the Relationship Between Experimental Conditions and Product Distribution in Electrophoretically Mediated Microanalysis (EMMA),” Montes, R.; Gomez, F. A.; Hanrahan, G., Electrophoresis, 2008, 29, 375-380.
59. “Through-a-Chip Affinity Capillary Electrophoresis to Estimate Binding Constants Between Receptors and Ligands”, Brown, A. L.; Morales, C.; Gomez, F. A. Talanta, 2008, 74, 605-612.
58. "Voltage Gradient Partial Filling Multiple Injection Affinity Capillary Electrophoresis to Estimate Binding Constants of Receptors to Ligands", Ramirez, A.; Gomez, F. A. J. Cap. Elect. Microchip Tech. 2007, 10, 43-50.
57. “Design and Development of a Flow Injection-Capillary Electrophoresis (FI-CE) Analyzer Employing Fiber Optic Detection.” Hanrahan, G.; Tse, F.; Dahdouh, F. T.; Clarke, K.; Gomez, F. A. J. Cap. Elect. Microchip Tech. 2007, 10, 1-6.
56. “Simple Fabrication of Fritless Chromatographic Microchips Packed with Conventional Reversed-Phase Silica Particles,” Gaspar, A.; Piyasena, M. E.; Gomez, F. A. Anal. Chem. 2007, 79, 7906-7909.
55. "Implementation of Chemometric Methodology in Affinity Capillary Electrophoresis (ACE): Predictive Investigation of Protein-Ligand Binding”, Hanrahan, G.; Montes, R.; Pao, A.; Johnson, A.; Gomez, F. A. Electrophoresis, 2007, 28, 2853-2860.
54. “Design and Fabrication of Chemically Robust Three-Dimensional Microfluidic Valves,” Maltezos, G.; Garcia, E.; Hanrahan, G.; Gomez, F. A.; Vjawhare, S.; van Dam, R. M.; Chen, Y.; Scherer, A. Lab Chip, 2007, 7 1209-1211.
53. "Determination of Binding Constants of Polyethylene Glycol Vancomycin Derivatives to Peptide Ligands Using Affinity Capillary Electrophoresis," Hernandez, L.; Hanrahan, G.; Rudolph, M.; Lammertink, R.; Kornfield, J.; Gomez, F. A. Chromatographia, 2007, 65, 299-303.
52. “1-[Ferrocenyl(hydroxy)methyl]-1,7-dicarba-closo-dodecaborane: Synthesis and X-ray Crystal Structure.” Fields, J.; Ouyang, X.; Herron, S. R.; Kantarjieff, K. A.; Jabalameli, A.; Gomez, F. A. J. Chem. Crystallogr. 2007, 37, 55-62.
51. “Partial-Filling Multiple-Injection Affinity Capillary Electrophoresis (PFMIACE) to Examine Binding Constants of Receptors to Ligands,” Zavaleta, J.; Chinchilla, D.; Ramirez, A.; Pao, A.; Martinez, K.; Nilapwar, S.; Ladbury, J. E.; Mallik, S.; Gomez, F. A., Talanta, 2007, 71, 192-201.
50. “Partial Filling Affinity Capillary Electrophoresis Techniques to Probe the Binding of Glycopeptide Antibiotics to D-Ala-D-Ala Terminus Peptides”, Zavaleta, J.; Chinchilla, D. B.; Kaddis, C. F.; Martinez, K.; Brown, A.; Gomez, A.; Pao, A.; Ramirez, A.; Nilapwar, S.; Ladbury, J. E.; Gomez, F. A. J. Cap. Elect. Microchip Tech., 2006, 9, 101-117.
49. “Multiple-Injection Affinity Capillary Electrophoresis.” Zavaleta, J.; Chinchilla, D.; Ramirez, A.; Calderon, V.; Gomez, F. A. LCGC, 2006, 24, 1118-1132; 2007, 84-92.
48. “Multiple-Injection Affinity Capillary Electrophoresis to Examine Binding Constants Between Glycopeptide Antibiotics and Peptides", Zavaleta, J.; Chinchilla, D.; Martinez, K.; Gomez, F.A. J. Chromatogr. A, 2006, 1105, 59-65.
47. “Recent Developments in Affinity Capillary Electrophoresis. A Review”, Zavaleta, J.; Chinchilla,. D.; Brown, A.; Sogomonyan, T.; Ramirez, A.; Calderon, V.; Gomez, F. A. Curr. Anal. Chem. 2006, 2, 35-42.
46. “Multiple-Injection Affinity Capillary Electrophoresis to Estimate Binding Constants of Receptors to Ligands”, Chinchilla, D.; Zavaleta, J.; Martinez, K.; Gomez, F. A. Anal. Bioanal. Chem. 2005, 383, 625-631.
45. “Flow Injection-Capillary Electrophoresis (FI-CE): Recent Advances and Applications", Hanrahan, G.; Dahdouh, F.; Clarke, F.; Gomez, F.A. Curr. Anal. Chem. 2005, 1, 321-328.
44. "Optimization of Conditions for Flow-Through Partial-Filling Affinity Capillary Electrophoresis to Estimate Binding Constants of Ligands to Receptors", Brown, A.; Desharnais, R.; Roy, B.C.; Malik, S; Gomez, F. A. Anal. Chim. Acta, 2005, 540, 403-410.
43. “Use of a Dual-marker Form of Analysis to Estimate Binding Constants Between Receptors and Ligands by Affinity Capillary Electrophoresis”, Villareal, V.; Brown, A.; Gomez, A.; Silverio, C.; Gomez, F. A., Chromatographia, 2004, 60, 73-78.
42. “Estimation of Binding Constants between Ristocetin and Teicoplanin to Peptides Using On-Column Ligand Derivatization Coupled to Affinity Capillary Electrophoresis”, Azad, M.; Brown, A.; Silva, I.; Gomez, F. A. Anal. Bioanal. Chem. 2004, 379, 149-155.
41. “Estimation of Binding Constants for the Substrate and Activator of Rhodobacter sphaeroides ADP-Glucose Pyrophosphorylase Using Affinity Capillary Electrophoresis”, Kaddis, J.; Zurita, C.; Moran, J.; Borra, M.; Polder, N.; Meyer, C. R.; Gomez, F. A. Anal. Biochem, 2004, 327, 252-260.
40. “Partial-Filling Techniques for Affinity Capillary Electrophoresis to Probe Receptor-Ligand Interactions”, Brown, A.; Silva, I.; Chinchilla, D.; Hernandez, L.; Gomez, F. A. LCGC Europe, 2004, 1-7.
39. “On-Column Synthesis Coupled to Affinity Capillary Electrophoresis to Determine Binding Constants of Peptides to Glycopeptide Antibiotics”, Azad, M.; Silverio, C.; Zhang, Y.; Villareal, V.; Gomez, F. A. J. Chromatogr., A, 2004, 1027, 193-204.
38. “Partial-Filling Affinity Capillary Electrophoresis”, Villareal, V.; Kaddis, J.; Azad, M.; Zurita, C.; Silva, I.; Hernandez, L.; Rudolph, M.; Moran, J.; Gomez, F. A. Anal. Bioanal. Chem. 2003, 376, 822-831.
37. "On-Column Derivatization and Analysis of the Antibiotics Teicoplanin and Ristocetin Coupled to Affinity Capillary Electrophoresis”, Silverio, C. F.; Azad, M.; Gomez, F. A. Electrophoresis, 2003, 24, 808-815.
36. “Determination of Binding Constants Between the Antibiotic Ristocetin A and D-Ala-D-Ala Terminus Peptides by Affinity Capillary Electrophoresis”, Azad, M.; Hernandez, L.; Plazas, A.; Rudolph, M.; Gomez, F. A. Chromatographia, 2003, 57, 339-344.
35. “Separation of DNA by Capillary Electrophoresis in Uncoated Silica Columns Using Hydroxypropylmethyl Cellulose as the Sieving Matrix”, Villareal, V.; Zhang, Y.; Zurita, C.; Moran, J.; Silva, I.; Gomez, F. A. Anal. Lett. 2003, 36, 451-463.
34. “Flow-Through Partial-Filling Affinity Capillary Electrophoresis Can Estimate Binding Constants of Neutral Ligands to Receptors Via a Competitive Assay Technique”, Kaddis, J.; Mito, E.; Heintz, J.; Plazas, A.; Gomez, F. A. Electrophoresis, 2003, 24, 1105-1110.
33. “Determination of Binding Constants Between Teicoplanin and D-Ala-D-Ala Terminus Peptides by Affinity Capillary Electrophoresis”, Silverio, C. F.; Plazas, A.; Moran, J.; Gomez, F. A. J. Liq. Chrom. & Rel. Tech. 2002, 25, 1677-1691.
32. “On-Column Enzyme-Catalyzed Microreactions Using Capillary Electrophoresis: Quantitative Studies,” Zhang, Y.; Kaddis, J.; Silverio, C.; Zurita, C.; Gomez, F. A. J. Cap. Elect. Microchip Tech. 2002, 7, 1-9.
31. “On-Column Ligand Synthesis Coupled to Partial-Filling Affinity Capillary Electrophoresis to Estimate Binding Constants of Ligands to a Receptor,” Zhang, Y.; Kodama, C.; Zurita, C.; Gomez, F. A. J. Chromatogr. A, 2001, 928, 233-241.
30. "Multiple-Step Ligand Injection Affinity Capillary Electrophoresis for Determining Binding Constants of Ligands to Receptors,” Zhang, Y.; Gomez, F. A. J. Chromatogr. A, 2000, 897, 339-347.
29. "On-Column Derivatization and Analysis of Amino Acids, Peptides, and Alkylamines by Anhydrides Using Capillary Electrophoresis,” Zhang, Y.; Gomez, F. A. Electrophoresis, 2000, 21, 3305-3310.
28. "Estimation of Receptor-Ligand Interactions by the Use of a Two-Marker System in Affinity Capillary Electrophoresis," Mito, E.; Zhang, Y.; Esquivel, S.; Gomez, F. A. Anal. Biochem. 2000, 280, 209-215.
27. "Use of Capillary Electrophoresis and Indirect Detection to Quantitate In-Capillary Enzyme-Catalyzed Microreactions,” Zhang, Y.; El-Maghrabi, R.; Gomez, F. A. Analyst, 2000, 125, 685-688.
26. "Flow-Through Partial-Filling Affinity Capillary Electrophoresis Can Estimate Binding Constants of Ligands to Receptors," Mito, E.; Gomez, F. A. Chromatographia, 1999, 50, 689-694.
25. "1-[Ferrocenyl(hydroxy)methyl]-1,2-dicarba-closo-dodecaborane," Crundwell, G.; Arellanes, C.; Gomez, F. A.; Kantardjieff, K. Acta Crystallogr., Sect. C, 1999, C55, IUC9900087.
24. "Optimization of Capillary Electrophoresis Conditions for In-Capillary Enzyme-Catalyzed Microreactions," Kwak, E. -S.; Esquivel, S.; Gomez, F. A. Anal. Chim. Acta, 1999, 397, 183-190.
23. "Use of a Partial-Filling Technique in Affinity Capillary Electrophoresis for Determining Binding Constants of Ligands to Receptors," Heintz, J.; Hernandez, M.; Gomez, F. A. J. Chromatogr. A, 1999, 840, 261-268.
22. "Use of Mobility Ratios to Estimate Binding Constants in Affinity Capillary Electrophoresis," Kawaoka, J.; Gomez, F. A. J. Chromatogr. B, 1998, 715, 203-210.
21. "The Use of Affinity Capillary Electrophoresis for Determining Binding Constants of Ligands to Receptors," Zhao, D. S.; Kwak, E. -S.; Kawaoka, J.; Esquivel, S.; Gomez, F. A. Am. Lab. 1998, 30, 40-47.
20. "Double Enzyme-Catalyzed Microreactors Using Capillary Electrophoresis," Zhao, D. S.; Gomez, F. A. Electrophoresis 1998, 19, 420-426.
19. "Enzyme-Catalyzed Microreactions Using Capillary Electrophoresis: A Quantitative Study," Zhao, D. S.; Gomez, F. A. Chromatographia 1997, 44, 514-520.
18. "Determination of the Binding of -Cyclodextrin Derivatives to Adamantane Carboxylic Acids Using Capillary Electrophoresis," Kwak, E. -S.; Gomez, F. A. Chromatographia 1996, 43, 659-662.
17. "Carboracyles: Macrocyclic Compounds Composed of Carborane Icosahedra Linked by Organic Bridging Groups," Jiang, W.; Chizhevsky, I. T.; Mortimer, M. D.; Chen, W.; Knobler, C. B.; Johnson, S. E.; Gomez, F. A.; Hawthorne, M. F. Inorg. Chem. 1996, 35, 5417-5426.
16. "Multiple-Plug Binding Assays Using Affinity Capillary Electrophoresis," Gomez, F. A.; Mirkovich, J. N.; Dominguez, V. M.; Liu, K. W.; Macias, D. M. J. Chromatogr. A, 1996, 727, 291-299.
15. "Determination of the Binding of Ligands Containing the N-2,4-dinitrophenyl Group to Bivalent Monoclonal Rat anti-DNP Antibody Using Affinity Capillary Electrophoresis," Mammen, M.; Gomez, F. A.; Whitesides, G. M. Anal. Chem. 1995, 67, 3526-3535.
14. "Using Capillary Electrophoresis to Follow the Acetylation of the Amino Groups of Insulin and to Estimate their Basicities," Gao, J.; Mrksich, M.; Gomez, F. A.; Whitesides, G. M. Anal. Chem. 1995, 67, 3093-3100.
13. "Determination of the Net Charge of Proteins Using Capillary Electrophoresis," Gao, J.; Gomez, F. A.; Haerter, R.; Whitesides, G. M. Proc. Natl. Acad. Sci. U.S.A., 1994, 91, 12027-12030.
12. "Affinity Capillary Electrophoresis: Insights into the Binding of SH3 Domains by Peptides Derived from an SH3-Binding Protein," Gomez, F. A.; Chen, J. K.; Tanaka, A.; Schreiber, S. L.; Whitesides, G. M. J. Org. Chem. 1994, 59, 2885-2886.
11. "Determination of Binding Constants of Ligands to Proteins by Affinity Capillary Electrophoresis: Compensation for Electroosmotic Flow," Gomez, F. A.; Avila, L. Z.; Chu, Y. -H.; Whitesides, G. M. Anal. Chem. 1994, 66, 1785-1791.
10. "Carboracycles: A Family of Novel Macrocyclic Carborane Derivatives," Chizhevsky, I. T.; Johnson, S. E.; Knobler, C. B.; Gomez, F. A.; Hawthorne, M. F. J. Am. Chem. Soc. 1993, 115, 6981-6982.
9. "Organofunctionalized Derivatives of O-Carborane as Precursors to Non-Oxide Ceramics of Boron," Johnson, S. E.; Gomez, F. A.; Hawthorne, M. F.; Thorne, K. J.; MacKenzie, J. D. Eur. J. Solid State & Inorg. Chem. 1992, 29, 113-125.
8. "Synthesis and Structural Characterization of Pyrazole Bridged Metalla-bis(dicarbollide Derivatives of Cobalt, Nickel, Copper, and Iron: Models for Venus Flytrap Cluster Reagents," Varadarajan, A.; Johnson, S. E.; Gomez, F. A.; Chakrabarti, S.; Knobler, C. B.; Hawthorne, M. F. J. Am. Chem. Soc. 1992, 114, 9003-9011.
7. "Synthesis and Structural Characterization of Metallacarboranes Containing Bridged Dicarbollide Ligands," Gomez, F. A.; Johnson, S. E.; Knobler, C. B.; Hawthorne, M. F. Inorg. Chem. 1992, 31, 3558-3567.
6. "A Simple Route to C-Monosubstituted Carborane Derivatives," Gomez, F. A.; Hawthorne, M. F. J. Org. Chem. 1992, 57, 1384-1390.
5. "A Versatile Protecting Group for 1,2-Dicarba-closo-dodecaborane(12) and the Structure of a Silylcarborane Derivative," Gomez, F. A.; Johnson, S. E.; Hawthorne, M. F. J. Am. Chem. Soc. 1991, 113, 5915-5917.
4. "The Use of Mixed Halogens, ICl and IBr, and (C2H5)2NSF3 as Halogenating Agents for Closo-2,4-C2B5H7 and Some Derivatives," Gomez, F. A.; Onak, T.; Arias, J.; Alfonso, C. Main Group Metal Chemistry 1990, 13(4), 237-246.
3. "The Latino Science Recruitment Project," Gomez, F. A. J. Chem. Ed. 1990, 67, 318-320.
2. "Conversion of closo-2,4-C2B5H7 to [nido-2,4-C2B5H7]-," Abdou, Z. J.; Gomez, F.; Abdou, G.; Onak, T. Inorg. Chem. 1988, 27, 3679-3680.
1. "Synthetic and Rearrangement Studies on the Carboranes B-X-closo-2,4-C2B5H6 (X=Br, I) and B,B'-X2-closo-2,4-C2B5H5. Correlation of B-Halo- and B,B'-Dihalodicarba-closo-heptaborane Isomer Stabilities," Ng, B.; Onak, T.; Gomez, F.; DiStefano, E. W. Inorg. Chem. 1985, 24, 4091-4096.
Books
1. "Biological Applications of Microfluidics," Gomez, F. A. ed., John Wiley & Sons, Inc., 2008.
2. "Chemometrics in Capillary Electrophoresis," Hanrahan, G.; Gomez, F. A. eds., John Wiley & Sons, Inc., 2009.
Book Chapters
1. Affinity Capillary Electrophoresis to Examine Receptor-Ligand Interactions. Azad, M.; Kaddis, J.; Villareal, V.; Hernandez, L.; Silverio, C. S.; Gomez, F. A. In Methods in Molecular Biology, Humana Press, 2004, pp 153-168.
2. Applications of Capillary Electrophoresis to Molecular Recognition and Analysis of In-Capillary Enzyme-Mediated Transformations. Zavaleta, J.; Chinchilla, D.; Brown, A.; Ramirez, A.; Calderon, V.; Sogomonyan, T.; Montes, R.; Gomez, F. A. In Advances in Chromatography, CRC Press, 2006, pp 125-172.
3. Microfluidics. Gomez, F. A. In Biological Applications of Microfluidics, Gomez, F. A. Ed. John Wiley & Sons, Inc., 2008, pp. 1-7.
4. Chemical Separations in 3D Microfluidics. Maltezos, G. M.; Gomez, A.; Gomez, F. A.; Scherer, A. In Biological Applications of Microfluidics; Gomez, F. A., Ed. John Wiley & Sons, Inc., 2008, pp. 263-272.
5. Magnetic Bead-Based Methods to Study the Interaction of Teicoplanin with Peptides and Bacteria. Piyasena, M. E.; Gomez, F. A. In Biological Applications of Microfluidics; Gomez, F. A., Ed. John Wiley & Sons, Inc., 2008, pp. 473-488.
6. On-Column Ligand/Receptor Derivatization Coupled to Affinity Capillary Electrophoresis. Zavaleta, J.; Chinchilla, D.; Gomez, A.; Sogomonyan, T.; Silverio, C.; Azad, J. Gomez, F. A. In Methods in Molecular Biology, Humana Press, 2008, pp. 647-660.
7. Chemometrical Experimental Design-Based Optimization Studies in Capillary Electrophoresis Applications. Montes, R.; Riveros, T. A.; Dahdouh, F.; Hanrahan, G.; Gomez, F. A. In Chemometrics in Capillary Electrophoresis, Hanrahan, G. and Gomez, F. A. eds., John Wiley & Sons, Inc., 2010, pp. 75-92.
8. Microfluidics in Protein Chromatography. Gomez, F. A. In Protein Chromatography: Methods and Protocols in Methods in Molecular Biology, Loughgan, S. and Walls, D., Eds. Humana Press, 2011, pp. 137-150..
9. Microchip Capillary Electrophoresis to Study the Binding of Ligands to Teicoplanin Derivatized on Magnetic Beads. Riveros, T. A.; Lo, R.; Salgado, M.; Carmona, H.; Gomez, F. A. In Capillary Electrophoresis and Microchip Capillary Electrophoresis, Garcia, C. D. and Carrilho, E., Eds. John Wiley & Sons, Inc., 2013, pp. 359-366.
Recently published or submitted:
- “Enzyme Chemotaxis on Paper-Based Devices”, Ilacas, G. C.; Basa, A.; Sen, A.; Gomez, F. A. Anal. Sci. 2018, 34, 115-119.
- “Enzyme-Linked Immunosorbent Assays (ELISA) Based on Thread, Paper, and Fabric”, Gonzalez, A.; Gaines, M.; Gallegos, L. Y.; Guevara, R.; Gomez, F. A. Electrophoresis, 2018, 39, 476-484.
- “Miniaturized Al/AgO Coin Shape and Self-Powered Battery Featuring Painted Paper Electrodes for Portable Applications”, Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2018, 273, 101-107.
- “Thread/Paper- and Paper-Based Microfluidic Devices for Glucose Assays Employing Artificial Neural Networks”, Lee, W.; Gonzalez, A.; Arguelles, P. N.; Guevara, R.; Gonzalez-Guerrero, M. J.; Gomez, F. A. Electrophoresis, 2018, 12, 1443-1451.
- “Thread/Paper-, and Fabric Enzyme-Linked Immunosorbent Assays (ELISA)”, Gonzalez, A.; Gaines, M.; Gallegos, L. Y.; Guevara, R.; Gomez, F. A. Methods, 2018, 146, 58-65.
- “A Microfluidic Glucose Sensor Incorporating a Novel Thread-Based Electrode System”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 2131-2135.
- “Microfluidic Thread-Based Electrode System to Detect Glucose and Acetylthiocholine”, Gaines, M.; Gonzalez-Guerrero, M. J.; Uchida, K.; Gomez, F. A. Electrophoresis, 2018, 39, 3082-3086.
- “Simple and Sensitive Colorimetric Assay System for Dopamine Using Microfluidic Paper-Based Analytical Devices”, Liu, C.; Gomez, F. A.; Miao, Y.; Cui, P.; Lee, W. Talanta 2019, 194, 171-176.
- “3D Multilayered Paper- and Thread/Paper-Based Microfluidic Devices for Bioassays”, Neris, N. M.; Guevara, R. D.; Gonzalez, A.; Gomez, F. A. Electrophoresis, 2019, 40, 296-303.
- “An Optimized Microfluidic Paper-Based NiOOH/Zn Alkaline Battery”, Burrola, S.; Gonzalez Guerrero, M. J.; Avoundjian, A.; Gomez, F. A. Electrophoresis, 2019, 40, 469-472.
- “Paper-Based Microfluidic Devices for Glucose Assays Employing a Metal-Organic Framework (MOF)”, Ilacas, G. C.; Basa, A.; Nelms, K.; Sosa, J.; Liu, Y.; Gomez, F. A. Anal. Chim. Acta, 2019, 1055, 74-80.
- “Microfluidic Paper-Based Analytical Devices (uPADs): Miniaturization and Enzyme Storage Studies”, Ilacas, G. C.; Gomez, F. A. Anal. Sci. 2019, 35, 379-384.
- "An All-Printed 3D-Zn/Fe3O4 Paper Battery", Gonzalez-Guerrero, M. J.; Gomez, F. A. Sens. Actuators, B Chem. 2019, 289, 226-233.
Recent talks and presentations:
- Microfluidic Thread-Based Electrode System to Detect Glucose and Acetylthiocholine. Uchida, K.; Gaines, M.; Gonzalez,-Guerrero, M. J.; Gomez, F. A. 31st Annual CSUPERB Symposium, Anaheim, CA, January, 2019.
- Cord-Based Microfluidic Chips as a Platform for ELISA and Glucose Assays. Elomaa, J.; Gallegos, L.; Gomez, F. A. 31st Annual CSUPERB Symposium, Anaheim, CA, January, 2019.
- 3D Multilayered Paper- and Thread/Paper-Based Microfluidic Devices for Bioassays. Neris, N; Guevara, R.; Gonzalez, A.; Gomez, F. A. 31st Annual CSUPERB Symposium, Anaheim, CA, January, 2019.
Group summer party (July, 2018).
Maya Horii, Alyssa Fernandez, and Lauren Duenas attending SCCUR at Cal State San Marcos in November 2019.