(T3) Use of Simple Materials for Novel Microfluidic-Based Applications
Cal State LA Faculty Participants: Frank A. Gomez, Yixian Wang, Yangyang Liu
Penn State Faculty Participants: Joshua Robinson, Ayusman Sen
The Gomez research group is engaged in developing fundamental and applied research in the area of microfluidics. An area of focus is on using simple materials to develop new applications that have potential to solving global problems. Gomez and his students collaborate with a number of research groups both at Cal State LA and outside the university. Below are listed some of these on-going projects.
Enzyme Chemotaxis on Paper-Based Devices
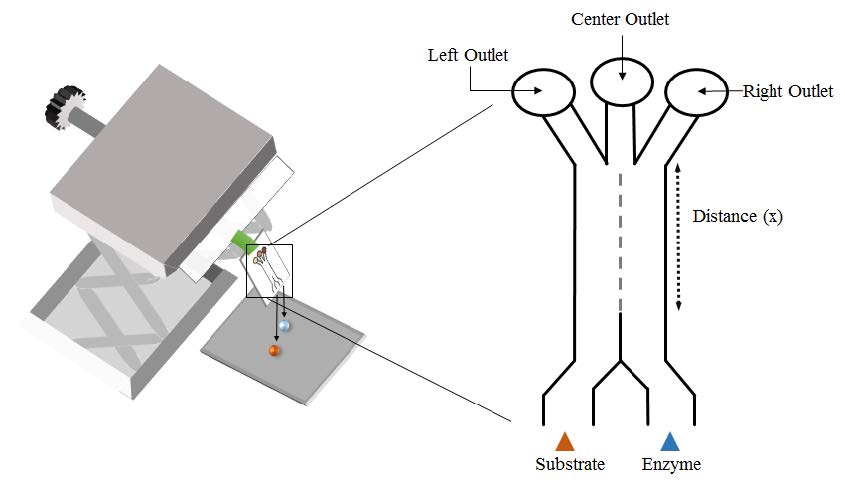
Microfluidics has served as a technology for the design and development of a myriad of devices owing to their reduced reagent consumption rate and short sampling-to-result time. Chemotaxis is the movement of materials, particularly biological species, in response to the influence of chemical stimulation. We have described, for the first time, chemotactic behavior on a microfluidic paper-based analytical device (μPAD) to afford a distribution of products not obtainable under other (non-μPAD) experimental conditions using as a model enzyme-substrate system glucose oxidase (GOx) and glucose. μPADs are easily fabricated by patterning hydrophobic materials in hydrophilic paper (Figure 1). They are low cost, compatible with biological samples, and have shown promise as platforms for various applications and in resource-limited settings.
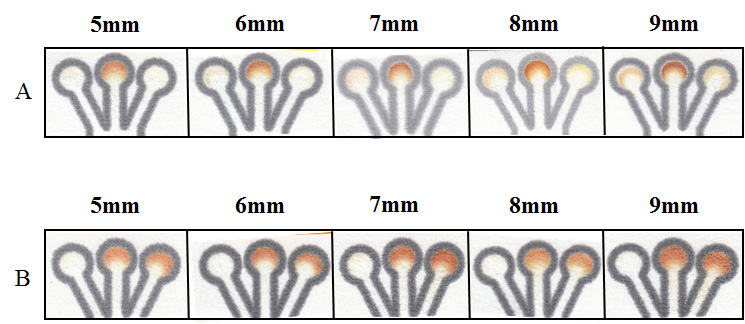
Figure 1. (Left) Schematics of experimental setup and µPAD used to observe chemotactic behavior. Chips were designed with mixing size areas (distance x) of 3 – 22, 25, and 30 mm. (Right) Visual detection of chemotactic movement. Mixture of KI:HRP is spotted in all outlets (circular regions in A and B) for colorimetric detection via presence of I2. (A) Experimental chips run with glucose (6 mM) and GOx (120 U/mL) in acetate buffer (0.2 M pH = 5.1). (B) Control chips run with acetate buffer (0.2 M pH = 5.1) and GOx (120 U/mL). Glucose was then run in each outlet lane to determine GOx movement independent of substrate interaction.
Thread-Based Microfluidic Sensors
Recently, thread and textiles have been utilized as potential inexpensive platforms for microfluidics and sensor applications. Hydrophilic threads are facile materials to transport aqueous fluid as they do not require external pumps. Thread is readily mass produced, easy to dispose of, and can be functionalized or coated from a myriad of materials. A number of applications have been successfully demonstrated using thread-based platforms typically using colorimetric detection. In order to achieve the sensitivities required to examine analytes at physiological levels, development of other detection techniques is warranted.
We have developed a reusable and simple to fabricate electrochemical sensor for the detection of glucose and acetylthiocholine using thread-based electrodes and nylon thread (Figure 2). The fabrication of the device consisted of two steps. First, three nylon-based electrodes (reference, working, and counter) were painted with one layer of conductive inks (silver and carbon ink, or silver/silver chloride ink). The electrodes were taped onto parafilm, and a piece of white nylon thread was wrapped around each electrode connecting the three electrodes.
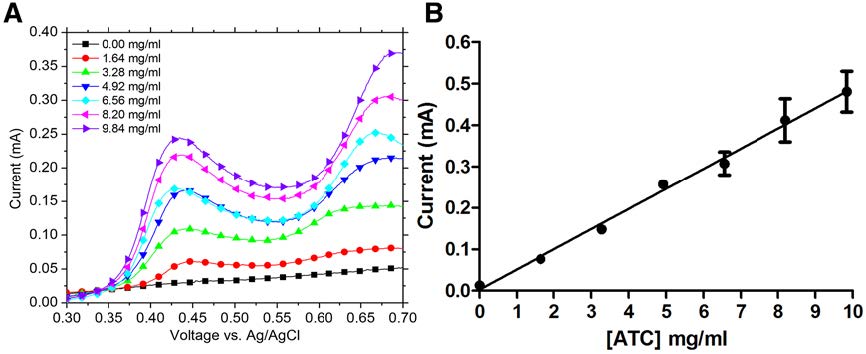
Figure 2. Left: A) Photograph of the thread-based electrochemical glucose sensor. Arrows indicate position of reference electrode (RE), working electrode (WE), and counter electrode (CE). Nylon thread is twisted around each electrode two (RE, WE) or three times (CE). B) Photograph of the thread-based electrochemical ATC sensor. Thread is twisted around each electrode three (RE, WE) or five times (CE). Right: A) Representative cyclic voltammograms for increasing ATC concentration. Oxidation peak occurred at 0.68 V. B) Calibration plot of the current output as a function of ATC concentration. Current output values were obtained from the linear sweep voltammograms at 0.68 V. Error bars correspond to the tests run in triplicate; each point represents the average of three values. R2 = 0.995 for the best fitting line.
Miniaturized Electrochemical Sensing Systems
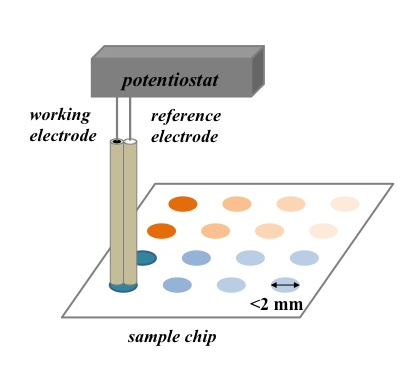
The Gomez and Wang groups are collaborating on developing a cost-efficient miniaturized electrochemical sensing system. Miniaturizing the sample size and decreasing the cost simultaneously is frequently a challenge in developing efficient personal diagnostic devices. In this project, the Wang group develops a dual electrode with a carbon ink barrel and a silver/silver chloride barrel as the working and reference electrodes, respectively. The dual electrode is then used to electrochemically detect various concentrations of analytes in solution dropped on arrays printed on microfluidic chips developed by the Gomez group. The current diameter of the electrode and the sample spots are 2 mm with a sample volume as small as 0.8 μL. The two groups are currently working on decreasing the electrode size and optimizing the sample spots, thereby, lowering the limit of detection.
Figure 3. Schematic of the miniaturized electrochemical sensor.
Nanoflowers as Sensors
In collaboration with Joshua Robinson at PSU, we have been using MoS2-based nanoflowers as biosensors (Figure 4). Preliminary work shows that these materials can be used in the development of glucose sensors.
Figure 4. Representative MoS2 nanoflower used in a glucose sensor.
Paper-Based Microfluidic Devices Employing Metal-Organic Frameworks (MOFs)
Biosensors have long been used to detect and quantify the presence of a biological species in a system and now include a myriad of materials. More recently, porous materials such as zeolites have been shown to possess unique properties that make them advantageous for sensing applications. Metal-organic frameworks (MOFs) are an exciting class of inorganic-organic hybrid materials consisting of metal ions connected by various organic linkers.
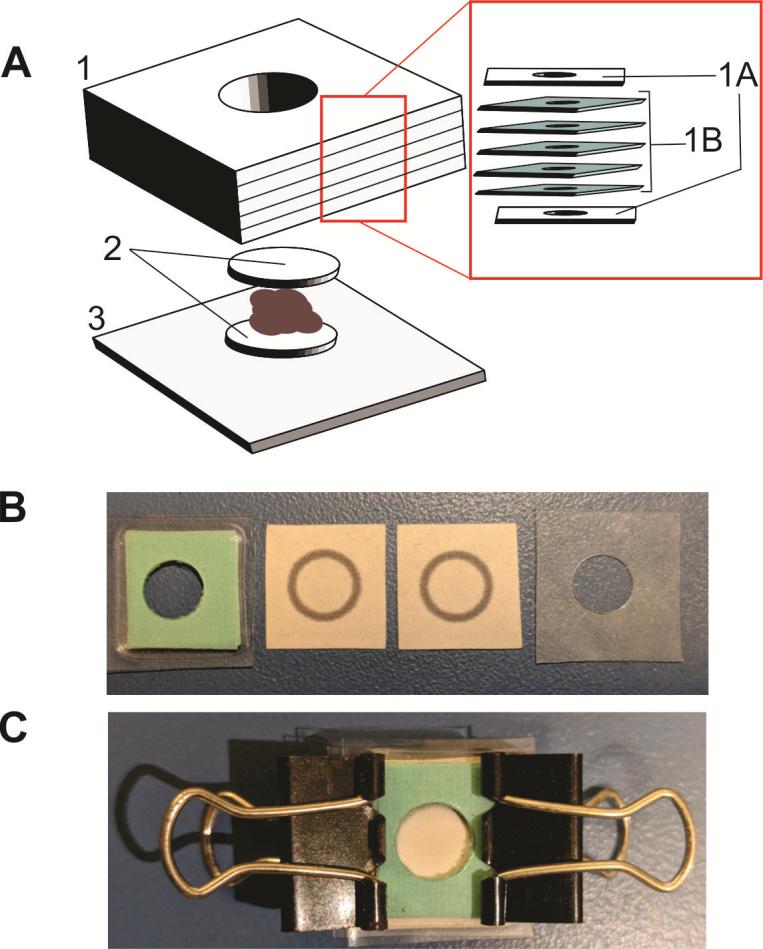
In collaboration with Yangyang Liu and her students (Cal State LA) we have developed two microfluidic paper-based analytical devices (μPADs) (Figure 5), one well-based and the other based on a lateral flow assay (LFA) configuration, to detect glucose via a colorimetric assay using the solid metal-organic framework (MOF) Zr-PCN-222(Fe), to encapsulate glucose oxidase (GOx). The devices yielded a correlation between intensity and glucose concentration. The development of these devices employing MOFs as biomimetic catalysts should further expand the applications of microfluidic technologies for sensors a variety of analytes.
Figure 5. (A) Schematic of the well-based μPAD showing layers 1-3: Layer 1. Paper well comprised of laminate sheets (1A), and five layers of wax-printed chromatography paper (1B); Layer 2. Chromatography chip design; Layer 3. Laminate support. (B) Well-based μPAD layers and (C) assembled chip.