(T2) Oxygen-evolving electrocatalysts and photoelectrocatalysts immobilized onto lightabsorbing perovskite metal oxides
CSULA Faculty Participants: Radi Jishi, Matthias Selke, Feimeng Zhou
Penn State Faculty Participants: Ismaila Dabo, Tom Mallouk, Venkatraman Gopalan
Oxygen-evolving electrocatalysts and photoelectrocatalysts immobilized onto light-absorbing perovskite metal oxides
We continue to synthesize and characterize light-absorbing stable semiconductive oxides for water splitting photoanodes. Employing hydrothermal method we synthesized a mixture of La2O3 and Fe2O3 nanoparticles. We have successfully converted the mixture into LaFeO3 perovskite at much lower temperatures that are suitable for its immobilization onto conductive glass slides and will use such slides as the photoanode.
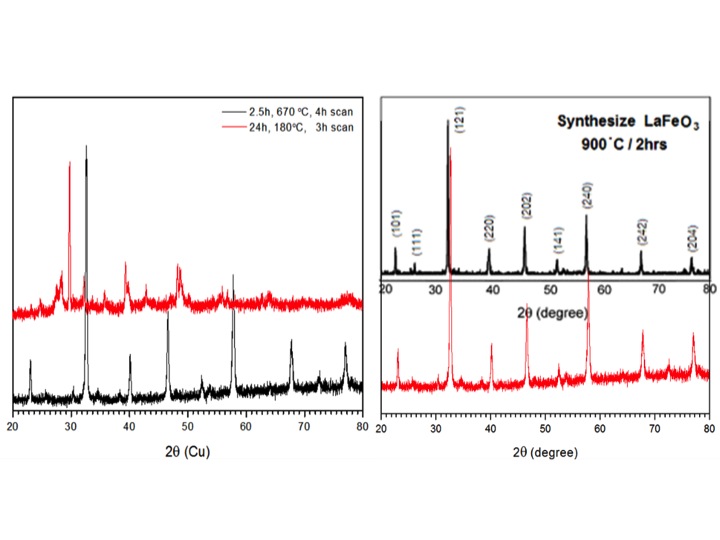
Figure 1. XRD diffraction patterns of as synthesized LaFeO3 mixture (top trace of left panel), calcinated at 670°C (bottom trace of left panel) and 900 °C (bottom trace of right panel), and reported perovskite (top trace of right panel, Haron, W.; Wisitsoraat, A.; Wongnawa, S. International Conference on Biotechnology, Nanotechnology and Environmental Engineering (ICBNE’15))
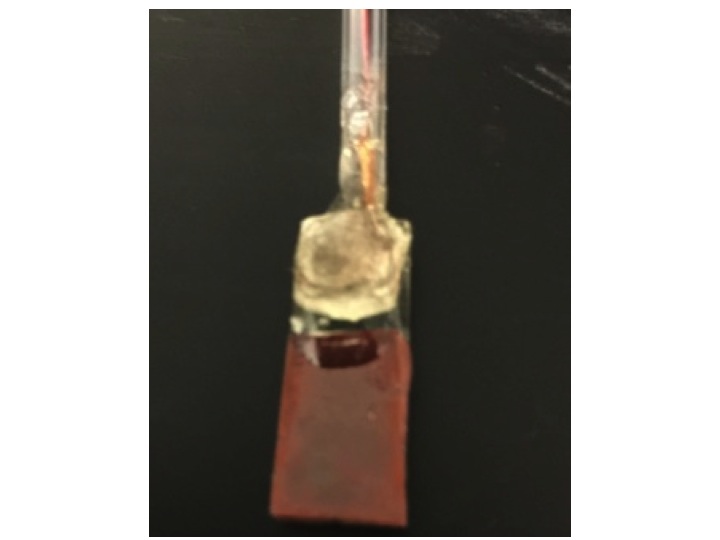
As shown in Figure 1, the as-synthesized mixture has a different XRD pattern from the reported, indicative of non-perovskite structure. However, calcination at 900 °C converted the oxides into the perovskite structure. More importantly, when calcinated at 670 °C, the mixture was also converted to perovskite structure. Usually, oxide perovskites are synthesized at temperatures much higher than the softening temperature of conductive glass. The low temperature synthesis of oxide perovskite is significant. Robust high quality perovskite semiconductive electrodes have been prepared as shown in Figure 2 (left panel); essential for practical applications.
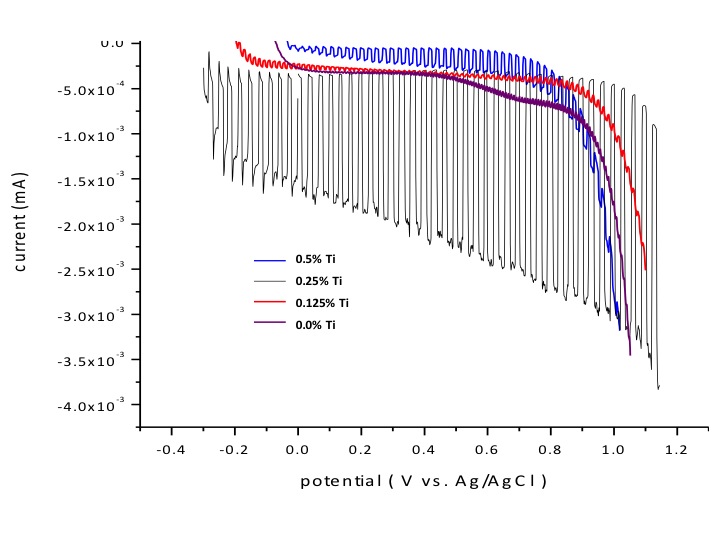
Figure 2. Left: A photograph of a LaFeO3 perovskite semiconductive electrode. Center: The anodic polarization curve of the LaFeO3 electrodes doped at different levels under the chopped light illumination. Right: Photo of LaCrO3 perovskite semiconductive electrode.
To serve as photoanode, the semiconductor has to be n-type conductive. We also synthesized and characterized the Ti-doped LaFeO3 perovskite film electrodes. As shown in the Center Panel of Figure 2, without doping the semiconductive electrode, little photocatalytic activity was observed. Increasing the doping level the electrode began to show the n-type photocatalytic activity toward water oxidation. The oxidation onset potential shifts dramatically. At 0.25% Ti relative to Fe, the optimal performance was attained. Significant photocatalytic oxidation current was observed and the onset oxidation potential shifts from ca. +1.1 to -0.3 V.
Although the LaFeO3 shows very good photocatalytic activity, its bandgap and edge are still not suitable for spontaneous splitting. We further tuned the bandgap and edge by synthesizing mixed metal oxide perovskite semiconductors LaFexCr1-xO3. Shown in the Right Panel of Figure 2 is the x=0, LaCrO3 perovskite film. Optimization and characterization is under way.
Transition-metal clusters and complexes
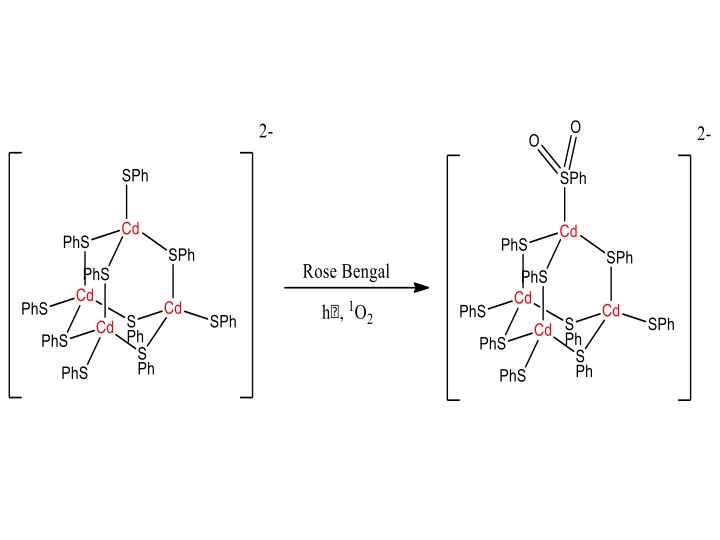
These species present a variety of potential applications ranging from imaging to photosensitization to the chemistry of clusters with singlet oxygen. Work on the photooxodation of Cd-Sulfur clusters and small nanoparticles continues. These clusters - such as Cd4(SPh)10 and Cd10S4(SPh)16 – are models for the surface of Cadmium-sulfur capped nanoparticles which have many different potential applications, including attachment of sensitizers to the Cadmium-sulfur surfaces. However, it is likely that energy transfer (generating singlet molecular oxygen) and/or electron transfer to the metal sulfur moieties lead to oxidation of the thiolate group and degradation of the nanoparticle. We have previously observed that the above-mentioned Cd-SR clusters quench singlet molecular oxygen with very large rate constants (> 108 M-1sec-1).
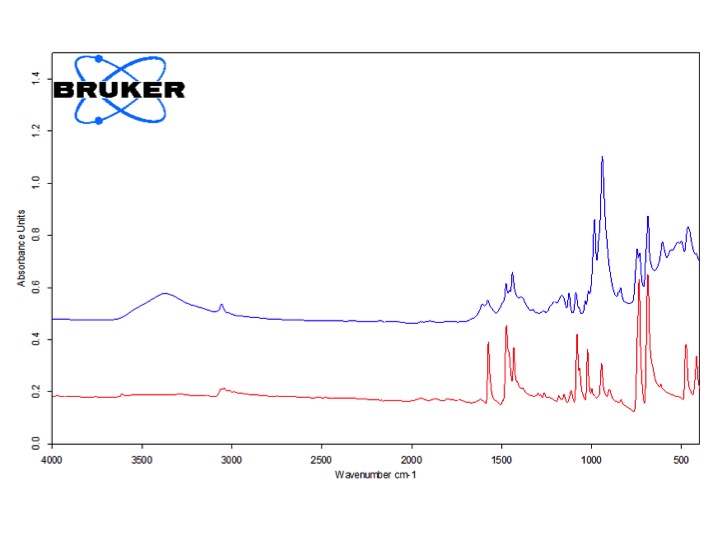
Figure 3: Left Panel: Scheme 1: Possible photooxidation of Cadmium-sulfur nanoclusters. Right Panel: Solid-state FTIR measurement of [Cd4(SPh)10]2- cluster (red) and [Cd4(SPh)10]2- cluster post Photooxidation (blue). Measurements done at PSU.
In collaboration with the materials characterization facility at PSU, we investigated if the quenching processes involve chemical reaction of singlet oxygen with the thiolate moieties, and what products are formed from these reactions. We have found that exposure of Cd4(SPh)10 to singlet oxygen leads to an insoluble oligomeric material. Detailed XPS and solid-state FTIR analyses at the materials characterization laboratory at PSUshows that ca. 80 % of the sulfur groups are oxidized to what appears to be O,O-bound sulfinates (Figure 3). We are currently trying to determine the rate constant for this chemical reaction in order to assess the main pathway by which singlet oxygen and the clusters interact. After this, we are planning to submit a joint publication reporting these findings which represent the first detailed study of the chemistry of singlet oxygen with Cadmium-sulfur clusters.
Diagnostic Tools with Fluorescent Nanoparticles
In collaboration with a group at Southeast University in Nanjing/China, the Selke group continues to study nanoclusters for bioimaging. They investigated highly fluorescent iron and zinc clusters for cancer imaging. In another ongoing project, the group studied platinum sulfur complexes for a variety of photochemical applications, including electron-transfer reactions and their ability to act as photosensitizers. The complexes contain a [Platinum(II)(bipyridine) moiety and various (N,S-aminoalkylthiolate) ligands. Some complexes undergo self-sensitized photooxidation while others do not. It is important to understand the factors that govern these processes, as this would ultimately determine future applications of such complexes. Characterization of the photooxidation products of these complexes such as metal bound sulfenates and sufinates, as a function of the availability of protons, in continuing. It appears that in protic solvents peroxidic intermediates can transfer oxygen atoms intermolecularly (leading to sulfenates) which is not the case in aprotic solvents.
Theoretical Materials Properties and Applications
Jishi with I. Dabo (PSU have been examining the usefulness of perovskites for hydrogen production and another project involving transition metal chalcogenide perovskites. Rodriguez has been working with colleagues at Wright-Patterson air force base to predict the structure and properties of chromium pnictide compound, and in the subsequent synthesis of this compound.